Bocox™
Erectile Dysfunction & Botulinum Toxin Type A
Uses Of Botulinum Toxin Type A
Botulinum Toxin Type A is a drug made from a toxin produced by the bacterium Clostridium botulinum. It's the same toxin that causes a life-threatening type of food poisoning called botulism. Doctors use it in small doses to treat health problems, including:
- Temporary smoothing of facial wrinkles and improving your appearance
- Severe underarm sweating
- Cervical dystonia - a neurological disorder that causes severe neck and shoulder muscle contractions
- Blepharospasm - uncontrollable blinking
- Strabismus - misaligned eyes
- Chronic migraine
- Overactive bladder
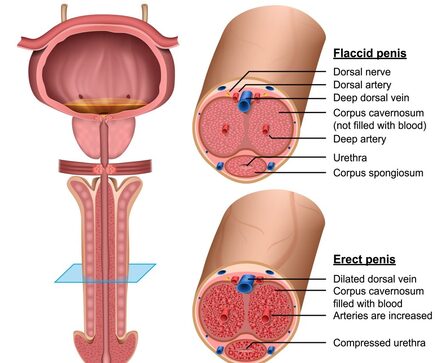
Botulinum Toxin Type A for Improvement of Penis Health and Male Sexual Function
It has been postulated that Botulinum Toxin Type A can be used in men suffering from Erectile Dysfunction, the mechanism is thought to be by blockade of the Sympathetic Nervous System, allowing increased activity of the Parasympathetic Nervous System which drives smooth muscle relaxation of the penile blood vessels, thereby allowing greater blood flow and increased penile hardness.
Note this is Off Label use for Botulinum Toxin Type A
It has been postulated that Botulinum Toxin Type A can be used in men suffering from Erectile Dysfunction, the mechanism is thought to be by blockade of the Sympathetic Nervous System, allowing increased activity of the Parasympathetic Nervous System which drives smooth muscle relaxation of the penile blood vessels, thereby allowing greater blood flow and increased penile hardness.
Note this is Off Label use for Botulinum Toxin Type A
|
In keeping with TAPS Approval 10287 (Therapeutic Advertising Pre-vetting Service) Pre-Post Treatment Advice, Outcomes and Duration of Effect are exempt from being advertised. This information can be discussed in a consultation with Dr Morunga.
Please refer to supplied evidence-based studies below of Botulinum Toxin Type A and Erectile Dysfunction. In addition, review information and evidenced based studies on the following Link Bocox® Provided by Dr Charles Runels Physician and Founder of the P Shot® The following research compares 50 units with 100 units of Botox for Erectile Dysfunction. |
Studies
1. Giuliano F, Joussain C, Denys P. Safety and Efficacy of Intracavernosal Injections of AbobotulinumtoxinA (Dysport®) as Add on Therapy to Phosphosdiesterase Type 5 Inhibitors or Prostaglandin E1 for Erectile Dysfunction—Case Studies. Toxins. 2019;11(5):283. doi:10.3390/toxins11050283
2. Abdelrahman IFS, Raheem AA, Elkhiat Y, Aburahma AA, Abdel-Raheem T, Ghanem H. Safety and efficacy of botulinum neurotoxin in the treatment of erectile dysfunction refractory to phosphodiesterase inhibitors: Results of a randomized controlled trial. Andrology. 2022;10(2):254-261. doi:10.1111/andr.13104
3. El-Shaer W, Ghanem H, Diab T, Abo-Taleb A, Kandeel W. Intra-cavernous injection of BOTOX® (50 and 100 Units) for treatment of vasculogenic erectile dysfunction: Randomized controlled trial. Andrology. 2021;9(4):1166-1175. doi:10.1111/andr.13010
4. Emura F, Peura D. Interview with Barry J. Marshall. Winner of the Nobel Prize in Medicine for the Discovery of Helicobacter pylori. :8.
5. Giuliano F, Denys P, Joussain C. Effectiveness and Safety of Intracavernosal IncobotulinumtoxinA (Xeomin®) 100 U as an Add-on Therapy to Standard Pharmacological Treatment for Difficult-to-Treat Erectile Dysfunction: A Case Series. Toxins. 2022;14(4):286. doi:10.3390/toxins14040286
6. Habashy E, Köhler TS. Botox for Erectile Dysfunction. The Journal of Sexual Medicine. 2022;19(7):1061-1063. doi:10.1016/j.jsxm.2022.03.216
Safety of Botulinum Toxin Type A
1. Naumann M, Jankovic J. Safety of botulinum toxin type A: a systematic review and meta-analysis. Current Medical Research and Opinion. 2004;20(7):981-990. doi:10.1185/030079904125003962
2. Stephens ML, Balls M. LD50 Testing of Botulinum Toxin for Use as a Cosmetic. 2005;(2):5.
3. Bhatia KP, Munchau A, Thompson PD, et al. Generalised muscular weakness after botulinum toxin injections for dystonia: a report of three cases. Journal of Neurology, Neurosurgery & Psychiatry. 1999;67(1):90-93. doi:10.1136/jnnp.67.1.90
4. Frevert J. Content of Botulinum Neurotoxin in Botox®/Vistabel®, Dysport®/Azzalure®, and Xeomin®/Bocouture®. Drugs R D. 2010;10(2):67-73. doi:10.2165/11584780-000000000-00000
5. Dhaked RK, Singh MK, Singh P, Gupta P. Botulinum toxin: Bioweapon & magic drug. Indian J Med Res. 2010;132(5):489-503. Accessed September 1, 2022. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3028942/
6. Omprakash HM, Rajendran SC. Botulinum Toxin Deaths: What is the Fact? J Cutan Aesthet Surg. 2008;1(2):95-97. doi:10.4103/0974-2077.44169
7. Arnon SS, Schechter R, Inglesby TV, et al. Botulinum Toxin as a Biological WeaponMedical and Public Health Management. JAMA. 2001;285(8):1059-1070. doi:10.1001/jama.285.8.1059
8. Nigam PK, Nigam A. BOTULINUM TOXIN. Indian J Dermatol. 2010;55(1):8-14. doi:10.4103/0019-5154.60343
9. BOTOX 100 Units – Summary of Product Characteristics (SmPC) – (emc). Accessed September 1, 2022.
1. Giuliano F, Joussain C, Denys P. Safety and Efficacy of Intracavernosal Injections of AbobotulinumtoxinA (Dysport®) as Add on Therapy to Phosphosdiesterase Type 5 Inhibitors or Prostaglandin E1 for Erectile Dysfunction—Case Studies. Toxins. 2019;11(5):283. doi:10.3390/toxins11050283
2. Abdelrahman IFS, Raheem AA, Elkhiat Y, Aburahma AA, Abdel-Raheem T, Ghanem H. Safety and efficacy of botulinum neurotoxin in the treatment of erectile dysfunction refractory to phosphodiesterase inhibitors: Results of a randomized controlled trial. Andrology. 2022;10(2):254-261. doi:10.1111/andr.13104
3. El-Shaer W, Ghanem H, Diab T, Abo-Taleb A, Kandeel W. Intra-cavernous injection of BOTOX® (50 and 100 Units) for treatment of vasculogenic erectile dysfunction: Randomized controlled trial. Andrology. 2021;9(4):1166-1175. doi:10.1111/andr.13010
4. Emura F, Peura D. Interview with Barry J. Marshall. Winner of the Nobel Prize in Medicine for the Discovery of Helicobacter pylori. :8.
5. Giuliano F, Denys P, Joussain C. Effectiveness and Safety of Intracavernosal IncobotulinumtoxinA (Xeomin®) 100 U as an Add-on Therapy to Standard Pharmacological Treatment for Difficult-to-Treat Erectile Dysfunction: A Case Series. Toxins. 2022;14(4):286. doi:10.3390/toxins14040286
6. Habashy E, Köhler TS. Botox for Erectile Dysfunction. The Journal of Sexual Medicine. 2022;19(7):1061-1063. doi:10.1016/j.jsxm.2022.03.216
Safety of Botulinum Toxin Type A
1. Naumann M, Jankovic J. Safety of botulinum toxin type A: a systematic review and meta-analysis. Current Medical Research and Opinion. 2004;20(7):981-990. doi:10.1185/030079904125003962
2. Stephens ML, Balls M. LD50 Testing of Botulinum Toxin for Use as a Cosmetic. 2005;(2):5.
3. Bhatia KP, Munchau A, Thompson PD, et al. Generalised muscular weakness after botulinum toxin injections for dystonia: a report of three cases. Journal of Neurology, Neurosurgery & Psychiatry. 1999;67(1):90-93. doi:10.1136/jnnp.67.1.90
4. Frevert J. Content of Botulinum Neurotoxin in Botox®/Vistabel®, Dysport®/Azzalure®, and Xeomin®/Bocouture®. Drugs R D. 2010;10(2):67-73. doi:10.2165/11584780-000000000-00000
5. Dhaked RK, Singh MK, Singh P, Gupta P. Botulinum toxin: Bioweapon & magic drug. Indian J Med Res. 2010;132(5):489-503. Accessed September 1, 2022. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3028942/
6. Omprakash HM, Rajendran SC. Botulinum Toxin Deaths: What is the Fact? J Cutan Aesthet Surg. 2008;1(2):95-97. doi:10.4103/0974-2077.44169
7. Arnon SS, Schechter R, Inglesby TV, et al. Botulinum Toxin as a Biological WeaponMedical and Public Health Management. JAMA. 2001;285(8):1059-1070. doi:10.1001/jama.285.8.1059
8. Nigam PK, Nigam A. BOTULINUM TOXIN. Indian J Dermatol. 2010;55(1):8-14. doi:10.4103/0019-5154.60343
9. BOTOX 100 Units – Summary of Product Characteristics (SmPC) – (emc). Accessed September 1, 2022.